| maps • volume xvi number 3 • Winter 2006-2007 |
MAPS’ 20th Anniversary Financial Report:
Fiscal Year June 1, 2005 to May 31, 2006
By Rick Doblin, Ph.D., MAPS President |
| |
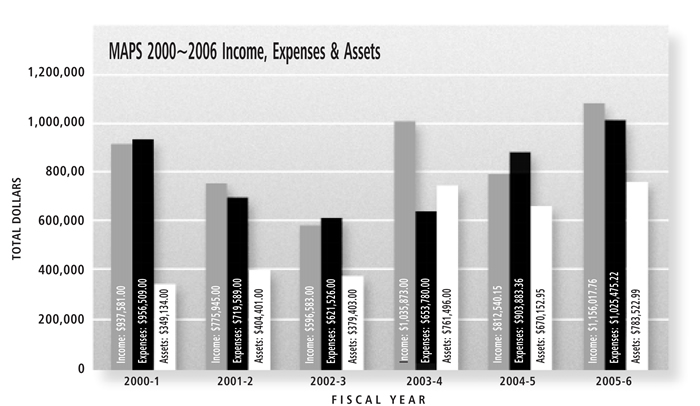
The Multidisciplinary Association for Psychedelic Studies (MAPS) came into legal existence on April 8, 1986, when I filed the Articles of Incorporation with the Florida Secretary of State. MAPS’ 20th anniversary passed on April 8, 2006, during MAPS’ Fiscal Year (FY) 2005-2006, which began on June 1, 2005 and ended on May 31, 2006. FY 05-06 was MAPS’ most productive year yet, concluding with more income ($1,156,017), expenses ($1,025,475), and assets ($783,522, of which $266,621 is restricted to specific projects) than in any previous year.
Furthermore, MAPS’ income in FY 2005-2006 does not include an additional $250,000 that philanthropist Peter Lewis pledged to donate directly to Harvard Medical School’s McLean Hospital, to support the MAPS-initiated, but no longer directly MAPS-sponsored, study of MDMA-assisted psychotherapy in subjects with anxiety associated with advanced-stage cancer (page 16).
MAPS is also benefiting from an increasing amount of donated labor of a highly skilled nature from people for whom salaries are not a necessity. This donated time is a crucial factor in MAPS’ ability to undertake the range of projects that it does and to implement its projects in a professional manner. MAPS now has a larger staff and more volunteers than ever, along with expanded opportunities to conduct research and contribute to public education. In addition, we recently coordinated our most ambitious gathering yet, the co-creation of Entheon Village at Burning Man, where we held MAPS’ 20th anniversary celebration (page 27).
MAPS is a research and educational organization that is, most essentially, a non-profit psychedelic and medical marijuana pharmaceutical company. Before presenting the nuts and bolts of FY 2005-2006, I’d like to offer a conceptual overview of what I consider to be the three basic stages of MAPS’ development. This overview will help situate the discussion of FY 2005-2006 in a longerterm perspective.
These three stages succinctly outline the original vision for MAPS. Stage 1: Low Maintenance/High Performance; Stage 2: High Maintenance/High Performance; and Stage 3: No Maintenance/High Performance. Stage 1 has taken MAPS the last two decades to complete, Stage 2 is likely to take the next decade, and Stage 3 will last for several decades or longer.
Stage 1: Low Maintenance/High Performance
During MAPS’ first two decades, after a great deal of sustained struggle, we became positioned to conduct the necessary research to develop psychedelics, but not yet marijuana, into legal prescription medicines in the US and abroad. We’ve helped contribute to a renaissance of psychedelic research around the world that has been forty years in the making. Psychedelic research teams around the world have obtained approval to conduct studies in human subjects using MDMA, MDE, psilocybin, mescaline, ketamine, ibogaine, DMT, and ayahuasca, but not yet LSD. This renaissance will fully arrive with the approval of LSD psychotherapy research. I expect this will occur in early 2007 in Switzerland, with the approval of Dr. Peter Gasser’s MAPS-sponsored study of LSD-assisted psychotherapy in subjects with anxiety associated with end-oflife issues. When completed, this will become the first LSD-assisted psychotherapy study in 35 years.
| |
| MAPS FY 2005-2006 |
| Income |
$1,156,017 |
| Expenses |
$1,025,475 |
| Net Change |
$130,542 |
| Net Assets beginning of FY |
$652,980 |
| Net Assets end of FY |
$783,522 |
| |
|
Asset Categories
|
|
| Assets: Restricted Funds – Liquid |
$266,621 |
| Assets: Unrestricted Funds – Liquid |
$476,901 |
| Assets: Remainder Interest in Home |
$40,000 |
| Total Assets |
$783,522 |
| |
|
| Income Categories |
|
| Donations from Individuals ³ $1000 |
$536,103 |
| Donations from Foundations ³ $1000 |
$277,465 |
| Donations from Individuals <$1000 |
$125,067 |
| Product sales: Books, Art, etc. |
$166,160 |
| Other Income: Interest, Conf. Fee |
$51,222 |
| Total Income |
$1,156,017 |
| |
|
| IRS 990 Expense Categories |
|
| Research Projects |
$247,156 |
| Educational Projects |
$310,183 |
| MAPS Bulletin, Website |
$65,271 |
| Project-Related Staff/Office Expenses |
$196,826 |
Product Costs/Royalties for Art
|
$84,134 |
| Management and General |
$116,593 |
| Fundraising |
$5,311 |
| Total Expenses |
$1,025,475 |
| |
|
The main reason that MAPS has been relatively low maintenance is that for the first twenty years of our existence, we’ve been almost entirely blocked in our efforts to conduct psychedelic and medical marijuana research by a seemingly endless series of political and scientific obstacles. Research intended to develop drugs into FDA-approved prescription medicines is expensive. In contrast, struggling and mostly failing to obtain permission for research is relatively inexpensive and takes only a few staff and minimal expenses for protocol development and literature reviews. There’s been extended periods of time when MAPS’ agenda has been so difficult to advance that I’d take time off during the days to paint our house, just so I could experience what it felt like to actually accomplish something.
Another factor in MAPS’ having remained low maintenance is that we’ve been able to leverage our funds to a substantial degree. Our most successful effort was helping turn $15,000 into $1.8 million, for the most methodologically well-designed study of the neurocognitive consequences of moderate to heavy Ecstasy use, reportedly the primary functional negative side effect of Ecstasy. We learned from a MAPS member about a unique population of people who had used MDMA but virtually no other drugs. We then invested $15,000 in a grant to Dr. John Halpern, Harvard Medical School’s McLean Hospital, for a pilot study with this population. Dr. Halpern used the data from the pilot study as the basis of a five-year, $1.8 million grant application to the National Institute on Drug Abuse (NIDA), which he was awarded. Previously, our record was leveraging $10,000 into $1 million. MAPS had invested $10,000, which we obtained from Peter Lewis, in protocol development and approval expenses incurred by Dr. Donald Abrams, UC San Francisco, for a study of marijuana in AIDS-wasting subjects. The study was approved by the FDA but NIDA refused to provide the marijuana. However, after several years, NIDA agreed to provide $1 million but for a study of marijuana’s risks in HIV+ subjects, which was the first study of the use of marijuana in a patient population in 15 years. We also worked with Dr. Abrams to leverage about $50,000 MAPS spent on vaporizer research into a $136,000 grant to Dr. Abrams from the California Center for Medicinal Cannabis Research (CMCR), for an FDA-approved clinical study in which vaporizers were compared to smoked marijuana.
The evidence supporting the rather bold claim that MAPS is a high performance organization is that we have managed to obtain permission for a series of psychedelic psychotherapy research projects around the world. Most importantly, our US MDMA/PTSD pilot study, conducted by Michael Mithoefer, M.D., and Annie Mithoefer, R.N., is about 3/4 complete and is generating remarkably promising results.
MAPS has been able to achieve high performance because we’ve been persistent, strategic, and have learned from our mistakes. Importantly, we’ve found enough joy and satisfaction in the struggle itself that our sustained lack of visible progress wasn’t demoralizing. We’ve also been fortunate to collaborate with a highly diverse and skilled community of volunteers and professionals who share our mission and sense of the importance of reintegrating into our culture the states of mind and the healing, creative and inspirational experiences that psychedelics and marijuana can facilitate. MAPS was ahead of its time for twenty years; now it feels like MAPS’ time has arrived.
MAPS came into existence in 1986 as a result of my prior work, beginning in 1984, helping to coordinate a lawsuit against the Drug Enforcement Administration (DEA) in an effort to oppose the criminalization of both the non-medical and the medical use of MDMA. As a result of the defeat of our effort to protect the therapeutic use of MDMA, I started MAPS to work toward developing MDMA, and also other psychedelics and marijuana, into FDA-approved prescription medicines. MAPS’ first stage of development is ending as we are finally initiating MDMA psychotherapy research in the US, Switzerland and Israel. Unfortunately, our medical marijuana research agenda is still fundamentally compromised by the federal monopoly on the supply of marijuana that can be used in FDA-approved research. Nevertheless, our lawsuit against the DEA has the potential to lead to the creation of our own privately-funded medical marijuana production facility at UMass Amherst, under the direction of Prof. Lyle Craker. If that potential is realized, we’ll have achieved the necessary prerequisites to justify the initiation of a major research effort aimed at developing the marijuana plant, either smoked or vaporized, into an FDAapproved prescription medicine (page 13). These two DEA lawsuits, two decades apart, form a pair of bookends to MAPS’ first stage of development.
Stage 2: High Maintenance/High Performance
We are now undertaking the delicate and thrilling task of transitioning into MAPS’ second stage of development, the high maintenance/high performance stage, and are experiencing the inevitable growing pains of expansion. Now that MAPS has obtained regulatory approval to actually start conducting scientific research with psychedelics, we have the rare opportunity to attempt to prove to the satisfaction of the FDA, regulatory agencies in Switzerland and Israel, and eventually the European Medicines Agency, that psychedelic-assisted psychotherapy can be safe and effective for some patient groups. If our lawsuit against the DEA is successful, which we will know any day now, we may also have the opportunity to develop marijuana into a prescription medicine.
For the next decade or so, give or take a few years, MAPS will need to increase its budget for research by about five to ten times, expand by about ten times the number of research teams, locations, and subjects, and probably double or triple our staff.
MAPS is now sponsoring a series of Phase 2 pilot studies into the risks and benefits of MDMA-assisted psychotherapy in subjects with treatment-resistant posttraumatic stress disorder (PTSD), with studies underway in the US (page 14), Switzerland (page 15), and Israel (page 17). In addition, MAPS is working on starting two additional MDMA/PTSD pilot studies in two other countries, Canada and Spain. (For a detailed strategic analysis of why MDMA in the treatment of PTSD is our top priority, I’ve written a Clinical Plan which can be found at www.maps.org/research/MDmaplan.html). These MDMA/PTSD pilot studies will take the next two years to complete and analyze, and will involve about 70 subjects. If the results are promising, we’ll then seek to initiate two large-scale multi-site Phase 3 studies, each with about 280 subjects, for a total of about 560 subjects. One of these Phase 3 studies will be conducted throughout the US and the other throughout Europe and Israel.
To supplement our MDMA/PTSD research, MAPS is initiating a parallel effort to study psychedelic psychotherapy as a treatment for anxiety associated with end-oflife issues. These studies build upon the pioneering research in the late 1960s and early 1970s into the use of LSD-assisted psychotherapy with cancer patients. As mentioned earlier, MAPS has initiated a study of MDMA-assisted psychotherapy in subjects with anxiety associated with advanced-stage cancer (page 16). We’re also working to sponsor clinical research with LSD in subjects with anxiety associated with end-of-life issues and are in the early stages of trying to develop a study of psilocybin in cancer patients with anxiety. These protocols are designed to lead to the study and practice of psychedelic psychotherapy in the dying process, in which a sequence of different psychedelics could be used, sometimes alone or even in combination, according to the clinical judgment of the therapists and the choices of the patients. These pilot studies will also be completed within about two years.
MAPS is diversifying its research by looking at two different patient populations and several different psychedelic substances. Before MAPS embarks on raising and spending $5 million on two large-scale Phase 3 studies, we will be able to compare results in subjects with PTSD and in subjects with anxiety associated with end-of-life issues. In this way, we can select the drug and patient combination that is most likely to result in regulatory approval for a psychedelic drug for a specific clinical indication. Of course, if the pilot studies in subjects with PTSD and in subjects with anxiety associated with end-of-life issues are all promising, we’ll try to go forward into Phase 3 studies for both indications.
In order to achieve our objectives of obtaining approval for psychedelics and marijuana as prescription medicines, MAPS needs to continue to transform into a high maintenance/high performance organization.
Stage 3: No Maintenance/High Performance
If all goes well during MAPS’ second stage of development, MAPS will have conducted sufficient research to persuade regulatory agencies to approve specific psychedelics and perhaps also marijuana as legal prescription medicines. MAPS will have emerged as a fully approved non-profit psychedelic (and hopefully marijuana) pharmaceutical company with multiple legal prescription medicines for sale. MAPS will manufacture (or contract out the manufacture) of these medicines and will sell them on a commercial basis as prescription medicines. MAPS will thus enter into a no maintenance/ high performance phase in which profits on the sale of our prescription medicines will be used to fund additional research designed to expand the number of our prescription drugs that are approved for an ever wider number of clinical indications. MAPS is thus one of the rare nonprofits that offers donors the potential of helping to create a self-sustaining organization.
 MAPS’ income from the sale of its prescription medicines will be limited, however, because MAPS will not have any patent rights on either the substances or their specific uses. As a result, the prescription medicines that MAPS will sell will be offered on a generic basis subject to competition from other manufacturers who will not have to replicate our research but will merely have to show that their drugs are chemical equivalents of the drugs that MAPS has obtained permission to market. Further limiting sales is the therapeutic model itself, since psychedelics are administered under supervision as adjuncts to psychotherapy, and would be ingested only a few times during a sustained period of mostly non-drug psychotherapy. This lack of patentability, along with the therapeutic model that doesn’t involve daily administration but only occasional psychedelic sessions, combined with the substantial “controversy” factor, has dissuaded any of the existing pharmaceutical companies from attempting to develop psychedelics into prescription medicines. Therein lies the opportunity for MAPS.
The financial potential of marijuana as a prescription medicine is a somewhat different story. Marijuana is often intended to be used on a daily basis for many years, for example in people with persistent conditions such as Multiple Sclerosis, chronic pain, HIV/AIDS, not to mention many others. As a result, the profit potential for marijuana as a medicine is likely to be substantially larger than for psychedelics. Nevertheless, profits will be limited because patients with a chronic disease have a long-term predictable use pattern and will have the financial incentive to either grow their own marijuana or have someone grow for them. Since MAPS is a non-profit organization, our goals would be to facilitate such uses through the sale of seeds and cloned plants instead of trying to maximize sales. Though it’s highly debatable, I think it’s likely that marijuana will be legalized within the next twenty years or so, further limiting the profit potential of medical marijuana. Furthermore, the pharmaceutical industry is researching a large number of marijuana extracts, isolated cannabinoids, and numerous patentable modifications of various cannabinoids, some of which may be superior medicines for certain specific indications.
During this third phase of MAPS’ organizational development, MAPS will also work to establish and develop our own chain of psychedelic clinics, where psychedelic medicines will be administered to patients. This is where the greatest potential lies for income, in that the fees paid for therapeutic services will be considerably higher than the cost of the drugs themselves. For example, an LSD session that can last 8 hours with two co-therapists could cost $1000 to $2000, while the LSD itself might cost $10 or less. There will be other organizations that also establish their own clinics, with MAPS both welcoming and assisting these organizations. Some of these organizations could be affiliated with particular religious groups, or run by people with a range of therapeutic methods and models. Despite both generic competition in the sale of our products and competition from other providers of psychedelic clinics, income from both the sale of MAPS’ legal medicines and the delivery of psychedelic medicines in our own clinics will generate sufficient funds to support additional research, to expand the range of medical uses and the scientific understanding of the human psyche, and to fund our public education efforts.
Stage 4 of MAPS’ development, if there is to be one, is for others to envision and implement. This Stage will be funded in part by the arrival, probably in 30 or 40 years, of $1 million (in today’s dollars) from the sale of a home in which MAPS currently has a remainder interest, left to MAPS in 1995 in a bequest by Eric Bass. Now, on to the nuts and bolts of MAPS FY 2005- 2006. For detailed information, MAPS’ IRS Form 990 is posted at www.maps.org/fiscal/990/2006.pdf
MAPS Income
MAPS’ income in FY 2005-2006 was $1,156,017, which can be divided into three different categories: 1) Donations and income for MAPS’ direct operational expenses and projects; 2) Sales of books, merchandise and art; and 3) Donations for projects conducted by other organizations, for which MAPS acts as fiscal sponsor. In FY 2005-2006, MAPS received donations of $824,574 for our operational expenses and projects and $166,160 from the sale of the MAPS Bulletin, books, and art (mostly portraits of Albert Hofmann by Dean Chamberlain and Alex Grey, signed by Albert). Of our remaining income, MAPS received donations of $165,266 for projects conducted by other people or organizations ($75,175 for SAFER’s educational projects, $62,004 for the Erowid web site, $18,409 for Alan Shoemaker’s Amazonian Shamanism Conference, $5,393 for a film about Stan Grof, and $4,285 for the Wo/Men’s Alliance for Medical Marijuana).
In terms of our total income, MAPS received $536,103 from individuals who donated $1000 or more, with the largest donations from John Gilmore ($245,000), Shawn Hailey ($60,000), a bequest by Lyn Ehrnstein ($35,524), Anonymous ($20,000), Rene Ruiz ($19,000), Michael Honack ($12,000), Mark Anderson ($11,282), Ami Shinitzky ($10,000), Joby Pritzker ($10,000), Susan Pritzker ($10,000), Robert Field ($10,000), Vanja Palmers ($10,000) and others. MAPS also received $277,465 from foundations that donated $1000 or more, with the largest donations from the Marijuana Policy Project ($135,475), Anonymous ($50,000), Anonymous ($40,000), the Audrey and Martin Gruss Foundation ($25,000), the Libra Foundation ($10,000), and others.
MAPS also received a total of $125,067 in unrestricted donations under $1000, from a total of about 1500 members. In 2007, we’re going to implement a direct mail campaign, with the goal of doubling our membership within two years.
MAPS Assets
Total assets at the end of FY 2005-2006 were $782,522. Of these assets, restricted funds amounted to $266,621 and unrestricted funds amounted to $516,901. Of the unrestricted funds, $40,000 is not liquid and is from the value of a remainder interest that MAPS was given in a $1 million home that will probably come to MAPS in 30 or 40 years. Since MAPS’ fiscal year runs from June 1–May 31, and since MAPS receives most of its unrestricted operational funds in December, these unrestricted assets include funds for operational costs that need to be spent through the remainder of 2006. A major value to MAPS of having unrestricted funds is that I can pledge to support certain research projects when they are still early in the protocol development and approval stage, encouraging research teams to work hard to obtain approval knowing that some funding will definitely be available if the study is fully approved. Then, once approved, I can try to raise funds specifically restricted to that research project, freeing up unrestricted funds for me to make pledges of support to new research teams. Of the restricted funds, $70,277 is for LSD/psilocybin research (raised mostly from the sale of Albert Hofmannsigned art by Dean Chamberlain and Alex Grey, to fund part of Dr. Gasser’s Swiss LSD/end-of-life anxiety study); $68,053 is for the Project Start-Up Fund: UMass Amherst (John Gilmore donated $100,000 to enable MAPS to fund initial costs of projects in early stages before other donors are likely to feel the projects are solid enough to justify support, ideally with the fund replenished if projects start); $64,692 is for the overall MDMA psychotherapy research effort; $44,498 is for the Harvard LSD/Psilocybin cluster headache study; $13,000 is for the marijuana vaporizer study (which we have been unable to conduct since NIDA refused to sell 10 grams for this study); $5,100 is for the Women’s Entheogen Fund; and $1000 is for a study examining creativity and psychedelics.
MAPS Expenses
MAPS’ cash expenses were $1,029,457, and expenses after depreciation according to IRS accounting rules amounted to $1,025,475. These expenditures are detailed in the expense summary chart on page 7. A fuller discussion of each line item is presented below.
In summary, MAPS spent $247,156 on research projects, $310,183 on educational projects, $65,271 on our Bulletin and website, and $196,826, for project-related staff and office expenses. MAPS also spent $84,134 in royalties and expenses for art and product sales, $116,593 on management and general expenses, and $5,311 on fundraising. Our largest research expense was the US MDMA/PTSD research project, on which we spent $161,448 for direct expenses.
As with most non-profit organizations, MAPS’ salaries are lower than those for jobs in the private-sector with comparable skills and responsibilities. However, as we grow, my goal is for MAPS to become able to afford competitive salaries. For a list of MAPS’ staff and the current salaries they earn, see the text below providing details about the salary category.
Conclusion
That’s the overview for FY 2005-2006; each of the expense categories are described below. Questions or comments are welcome. It’s a privilege to be able to do this work. I am profoundly grateful to all MAPS members, who pay the bills that empower your MAPS staff to engage in our truly worthy struggles. |
| SUMMARY 2005-2006 |
| |
|
| Research Projects |
|
| Ayahuasca EEG |
$594.13 |
| Ibogaine Follow-Up |
$15,594.53 |
| Iboga Therapy House |
$1,700.00 |
| MDE German Passie Couples Therapy |
$387.00 |
| MDMA Analysis (Ecstacy Pill Testing) |
$3,000.00 |
| MDMA Cancer/Halpern (Harvard) |
$4,311.11 |
| MDMA/Defense Mechanism |
$5,030.00 |
| MDMA Israel |
$19,337.50 |
| MDMA/NIMH Grant |
$4,132.00 |
| MDMA Lit Review |
$8,185.13 |
| MDMA PTSD-SC |
$161,448.95 |
| MDMA PTSD-Spain |
$317.82 |
| MDMA PTSD-Swiss |
$9,496.32 |
| MJ Production Facility/UMass Amherst |
$5,537.50 |
| Psilocybin/LSD Cluster Headache |
$8,084.50 |
| Research Projects Subtotal |
$247,156.00 |
| |
|
Education Projects
|
|
| Amicus Curiae Brief (Raich Medical Marijuana) |
$590.91 |
| Book-LSD My Problem Child |
$15,991.64 |
| Book-Secret Chief Revealed |
$66.00 |
| Book-The Ultimate Journey |
$5,850.80 |
| Burning Man 2005 |
$10,429.61 |
| Burning Man 2006 |
$2,370.45 |
| Conference-Basel/Hofmann |
$11,507.18 |
| Conference-Boom |
$1,515.53 |
| Conference-DPA |
$3,191.26 |
| Conference-Mindstates |
$5,000.00 |
| Conference Peru |
$18,409.22 |
| Conference Psytopia |
$1,824.00 |
| Conference-Sheshamans |
$970.19 |
| Erowid Website |
$62,004.76 |
| DEA/ALJ Lawsuit |
$46,625.48 |
| DEA/UMASS Cong. Sign on Letter |
$13,298.08 |
| Event-MPP Fundraising |
$1,000.00 |
| Film-Grof |
$5,393.13 |
| Film-Shulgin by Littlefield |
$50.00 |
| MAPS Forum |
$4,620.34 |
| MAPS Staff Retreat |
$400.00 |
| S.A.F.E.R./UC Boulder Colorado State |
$75,175.00 |
| Video-Difficult Trip Guidance |
$1,415.04 |
| Women's Alliance for
Medical Marijuana (WAMM) |
$4,285.00 |
| Women's Entheogen Fund |
$18,200.00 |
| Education Projects Subtotal |
$310,183.00 |
|
| |
|
| |
|
| MAPS Bulletin/Website/Internet |
|
| Bulletin |
$27,859.27 |
| Internet |
$40,000 |
| Web Administration |
$27,105.14 |
| Web Content/Res Page/Info@ |
$3,847.13 |
| MAPS Bulletin/Web Subtotal |
$65,271.00 |
| |
|
| Fundraising |
|
| MAPS Ads, Memb. Drive |
$5,311.37 |
| |
|
| Staff/Operating/Project-Related
and Management/General |
|
| Information |
$650.69 |
| Copies |
$776.04 |
| Phones |
$11,660.56 |
| Postal |
$13,823.97 |
Conference Fees
|
$570.00 |
| Management and General |
$116,593 |
| Staff Travel |
$25,773.01 |
| Salary & Benefits & Taxes |
$211,737.45 |
| Fees-Bank, Etc |
$4,258.16 |
| Equipment Rental |
$2,494.03 |
| Office Moving Expenses |
$3,430.00 |
| Office Rent Sarasota |
$8,259.24 |
| Office Rent Love Creek |
$12,000.00 |
| Office Supplies |
$6,996.74 |
| Computer/Office Equipment |
$4,704.11 |
| Overall Subtotal |
$313,419.00 |
| Project-Related Staff/Office Subtotal |
$196,826.00 |
| Management & General Subtotal |
$116,593.00 |
| |
|
| Product Cost/Royalties for Art |
|
| Books and Tapes |
$7,462.56 |
| Hofmann/Chamberlain Portrait (Regular) |
$21,606.92 |
| Hofmann/Chamberlain Portrait (Large) |
$15,500.15 |
| Huxley/Chamberlain Portrait |
$3,060.00 |
| Shulgin/Chamberlain |
$4,749.97 |
| Hofmann/Grey Portrait |
$31,754.75 |
| ProductCost/Royalties Subtotal |
$84,134.35 |
| |
|
| Grand Total |
$1,025,475.00 |
|
| Ayahuasca EEG - $594.13 |
| MAPS supported Frank Echenhoffer, Ph.D. for his study evaluating the efsfect of ayahuasca on EEG readings. (See the Spring 2005 MAPS Bulletin or www.maps.org/newsletters/ v15n1-html/eeg.html) |
| |
| Ibogaine Follow-Up - $15,594.53 |
| MAPS sponsored the protocol design and pilot testing of a study of the long-term outcome of opiate abusers treated with ibogaine at the Iboga Therapy House. The protocol has been approved by a Canadian Institutional Review Board (IRB). (See the Autumn 2006 MAPS Bulletin or www.maps.org/ibogaine) |
| |
| Iboga Therapy House - $1,700 |
| MAPS donated $1,700 to Vancouver’s Iboga Therapy
House for medical equipment. The Iboga Therapy House
will be one of the treatment sites for the MAPS-sponsored
follow-up study. (See www.ibogatherapyhouse.net) |
| |
| MDE Passie Couples Therapy - $387 |
| Torsten Passie, M.D., a German research psychiatrist, is in the protocol development and approval stage for a study of MDE (methelenedioxyethylamphetamine, a substance similar to MDMA) as an adjunct to traditional couples therapy. MAPS brought Dr. Passie to Israel to speak at a conference about MDMA and psychedelic research, which was attended by members of the Israeli Ministry of Health and Anti-Drug Authority. The conference helped MAPS obtain permission for the MDMA/PTSD research in Israel. (See the Summer 2005 MAPS Bulletin or www.maps.org/ news-letters/v15n2-html/conference.html) |
| |
| MDMA Analysis (Ecstacy Pill Testing) - $3,000 |
| The ecstasydata.org pill testing project, currently out of
funds, was co-sponsored by MAPS, DanceSafe, and
Erowid. It allowed people to anonymously send pills to a
DEA-licensed laboratory for analysis, with the results
posted online. (See the Spring 2005 MAPS Bulletin or
www.maps.org/news-letters/v15n1-html/testing.html) |
| |
| MDMA Cancer/Halpern (Harvard) - $4,311 |
| MAPS supported the protocol design and approval process for a study by John Halpern, M.D., Harvard Medical School, exploring the use of MDMA-assisted psychotherapy in the treatment of advanced-stage cancer patients with anxiety. (See article on page 16) |
| |
| MDMA/Defense Mechanism - $5,030 |
This ongoing study, conducted by Pål Johansen, licensed psychologist (NPF) and Ph.D. candidate at the Trondheim Psychotherapy Research Program at the Norwegian University of Science and Technology, and Teri S. Krebs, B.S., Program in Neuroscience, Boston University, analyzes audio and video recordings of therapy sessions from MAPS-sponsored studies of MDMA-assisted psychotherapy study, coding the patient’s use of a comprehensive set of psychological defense mechanisms. The objective of this study is to provide empirical evidence on how MDMA
influences behavior in the context of psychotherapy, to
understand how MDMA might facilitate the therapeutic
process, and finally to empirically inform the development
of a standardized treatment manual for MDMA-assisted
psychotherapy. (See the Winter 2005 MAPS Bulletin or
www.maps.org/news-letters/v15n3-html/
mdma_pilot_outcome.html) |
| |
| MDMA/PTSD Israel – $19,337 |
| These expenses were for a scientific conference in Israel on MDMA and psychedelic research, coordinated by MAPS and attended by members of the Israeli Ministry of Health and the Israeli Anti-Drug Authority, and for protocol development and approval expenses for a study evaluating MDMA-assisted psychotherapy as a treatment for terrorism- and war-related PTSD. This MDMA/PTSD study is fully approved and will be led by principal investigator Moshe Kotler, M.D., Chair of the Department of Psychiatry at the Sackler School of Medicine at Tel Aviv University and former chief psychiatrist of the Israeli Defense Forces. The male-female co-therapist team will consist of Rael Strous, M.D., and Rakefet Rodrigez, M.D., Though conducted in Israel, the study will also be submitted to FDA under MAPS’ Investigational New Drug (IND) application for MDMA and therefore fits into MAPS’ mission of developing MDMA as a prescription medicine approved by both the FDA and the European Medicines Agency. (See article on page 17) |
| |
| MDMA/NIMH Grant - $4,132 |
| MAPS funded the preparation of a grant to the National Institute of Mental Health (NIMH) for the development of a treatment manual for MDMA-assisted psychotherapy for PTSD. Not unexpectedly, the grant was rejected. Based on the reviewer’s comments, it is clear that we will need to wait until our US MDMA/PTSD study is completed and we have promising pilot data before resubmitting another grant application. (The application is posted at www.maps.org/research/mdma/ptsd_study/grant0605) |
| |
| MDMA Literature Review - $8,185 |
| MAPS Research Associate Ilsa Jerome, Ph.D., continued the ongoing review of all peer-reviewed scientific literature on MDMA published throughout FY 05-06. When applying to the FDA and Institutional Review Boards with a new protocol, it is necessary to have a comprehensive review of all factors related to risk. The literature review has been submitted as part of our Israeli and Swiss MDMA/PTSD protocols, and was a necessary part of those applications. (See www.maps.org/mdma/protocol/ litreview.html) |
| |
| MDMA/PTSD Study in South Carolina – $161,448 |
| The first FDA-approved study of the therapeutic use of MDMA, Dr. Michael Mithoefer’s ongoing Phase 2 MDMA/PTSD pilot study, was MAPS’ most strategically critical and costly research project in FY 05-06. This study will be completed in FY 06-07. Dr. Michael and Annie Mithoefer have been the core of our MDMA clinical research therapy training program, fulfilling a vital function by providing training for MAPS’ MDMA/PTSD research teams from Israel and Switzerland, and by contributing to the development of MAPS’ MDMA/PTSD treatment manual. The results of the study have been promising so far, with 17 out of 20 subjects enrolled. Dr. Mithoefer is especially seeking veterans with PTSD for the remaining subjects in his study. (See article on page 14) |
| |
| MDMA PTSD-Spain - $317 |
| Jose Carlos Bouso, Ph.D. candidate, is preparing to work on the design and approval process for a new version of his MAPS-sponsored MDMA/PTSD study, which was halted in 2002 due to political pressure. We are hopeful that since we have now obtained government approval for MDMA/PTSD studies in the US, Switzerland, and Israel, it will be politically feasible to resume research in Spain. Bouso’s original MDMA/PTSD protocol was the first government-approved MDMA psychotherapy study in the world, and treated six patients successfully. (See www.maps.org/mdma/spain/index.html) |
| |
| Swiss MDMA/PTSD - $9,496 |
| Dr. Peter Oehen’s MAPS- and Swiss Medical Assoc. for Psycholytic Therapy-sponsored MDMA/PTSD study has received full government approval from the Ethics Committee (Switzerland’s IRB equivalent), SwissMedic (Switzerland’s FDA equivalent), and BAG (Switzerland’s DEA equivalent). This study will also be submitted to FDA under MAPS’ Investigational New Drug (IND) application for MDMA in the treatment of PTSD. The first experimental session took place on October 19, 2006. (See article on page 15) |
| |
| MJ Production Facility/UMass Amherst - $5,537 |
| MAPS and Prof. Lyle Craker, Director of the Medicinal Plant Program at the UMass-Amherst Department of Plant, Soil and Insect Sciences, have been working since June 2000 to obtain a DEA Schedule I license for a MAPSsponsored medical marijuana production facility. Ending the six-decades-long government monopoly on the production of marijuana for research purposes is the key prerequisite to MAPS being able to justify sponsoring FDA clinical trials with marijuana to determine if it has the potential to be approved as a prescription medicine. During FY 05-06, our lawsuit against DEA for rejecting Prof. Craker’s application for a license was heard before a DEA Administrative Law Judge, attracting considerable attention from medical groups, politicians, and the media. The $5,537 was paid directly to UMass-Amherst for Prof. Craker’s time spent working on the application and lawsuit. MAPS obtained a grant from the Marijuana Policy Project for these funds. (See article on page 13) |
| |
| Psilocybin/LSD Cluster Headache - $8,084 |
| MAPS supported Andrew Sewell, M.D., and John Halpern, M.D., McLean Hospital, Harvard University, in collecting and analyzing hundreds of case reports detailing the use of LSD and/or psilocybin for treatment of cluster headaches. The reports were gathered from Erowid.org and Clusterbusters, an organization run by and for people with cluster headaches. In June 2006, Dr. Sewell published an article featuring these case reports in Neurology, the official journal of the American Academy of Neurology. Based on this data, MAPS assisted Drs. Sewell and Halpern in working on a protocol for a prospective study of psilocybin and LSD in people with episodic cluster headaches. (See www.maps.org/research/cluster/psilolsd/# cluster) |
| |
Amicus Curiae Brief
(Raich Medical Marijuana) - $590 |
| MAPS submitted Amicus Curiae briefs for Angel Raich’s Supreme Court case decided last June, and for her new “Right to Life” 9th Circuit Court case. (The briefs are posted at www.maps.org/mmj/mpp_amicus_11.30.05.pdf and www.maps.org/mmj/AvR101304.pdf) |
| |
| Book—LSD: My Problem Child - $15,991 |
| MAPS published a new edition of Dr. Albert Hofmann’s autobiography, which had been out-of-print for two decades. We timed its publication to coincide with the international symposium in honor of Dr. Hofmann’s 100th birthday in January 2006. Sales have been relatively swift. We also have a limited number of hardcover copies for sale, signed by Albert (See www.maps.org/catalog) |
| |
| Book—The Secret Chief Revealed - $66 |
| Royalties to author Myron Stolaroff. |
| |
| Book—The Ultimate Journey - $5,850 |
| These costs are for pre-production of Dr. Stanislav Grof’s latest book, The Ultimate Journey: Consciousness and the Mystery of Death. This includes staff time for editing, layout, and indexing. (See www.maps.org/catalog) |
| |
| Psychedelic Emergency Services at Boom -
$1,515 |
| The organizers of Boom Festival, which takes place in Portugal every other August, contracted with MAPS to provide psychedelic emergency services at this year’s festival. These expenses were for travel expenses for MAPS’ psychedelic emergency services team. Boom contributed $9,600 to MAPS, mostly in FY 06-07, plus tickets and food for 8 core staff members and 15 volunteers. |
| |
| Burning Man 2005 - $10,429 |
| MAPS developed its work assisting the Black Rock Rangers in staffing the Sanctuary tent and in providing experts in psychedelic research to speak at a lecture series. (See the Winter 2005 MAPS Bulletin or www.maps.org/newsletters/ v15n3-html/burningman.html) |
| |
| Burning Man: 2006 - $2,370 |
| MAPS held its 20th anniversary celebration at Burning Man in 2006. These expenses were for staff work on the lecture series and for coordinating psychedelic emergency services. (See article on page 27) |
| |
| Conference: Basel/Hofmann - $11,507 |
| The Spirit of Basel and the Gaia Media Foundation hosted a symposium, “LSD: Problem Child and Wonder Drug,” in honor of Dr. Albert Hofmann’s 100th birthday, bringing together over 2000 participants and presenters. MAPS brought several speakers to discuss topics related to psychedelic therapy, including Drs. Michael Mithoefer, John Halpern, Andrew Sewell, Charles Grob, and MAPS staffers Rick Doblin and Valerie Mojeiko. We also used this trip to coordinate with the Swiss MDMA/PTSD therapy team. (See the Spring 2006 MAPS Bulletin or www.maps.org/hofmann100/index.html) |
| |
| Conference: Drug Policy Alliance - $3,191 |
| Staff costs for travel and lodging at the November 2005 International Drug Policy Reform conference in Long Beach, CA, featuring nearly 1,000 participants and over 70 sessions. Rick Doblin represented MAPS in three session panels. MAPS Director of Communications Jag Davies and former MAPS staffers Julia Onnie-Hay and Falon Mihalic ran a MAPS information table, and the costs of their attendance were significantly reduced by Robert E. Field’s scholarship program for budding drug policy reformers. (See the Spring 2006 MAPS Bulletin or www.maps.org/news-letters/v15n4-html/ building_a_movement.html) |
| |
| Conference: Mindstates - $5,000 |
| MAPS staffers Rick Doblin, Julia Onnie-Hay, and Valerie Mojeiko ran an information booth and gave several presentations about MAPS’ strategy for psychedelic research development. Mindstates organizer Jon Hanna put together an excellent conference, but lost a significant amount of money. Since the conference served an important function, MAPS raised $10,000 to offset Jon’s debts. Shawn Hailey donated $5,000 directly to Jon, and $5,000 was donated by John Gilmore to MAPS, restricted for Jon. |
| |
| Conference: Peru - $18,409 |
| To help facilitate the Amazonian Shamanism Conference in July 2005 and July 2006, MAPS processed credit card orders on their behalf and forwarded 100% of ticket sales received to the conference organizers. In exchange, Soga del Alma donated a free conference registration (value $250) to MAPS staffer Julia Onnie-Hay, who gave a presentation about MAPS and ran a MAPS information table at the conference. |
| |
| Conference: Psytopia - $1,824 |
| Travel expenses for MAPS staffer Valerie Mojeiko to attend the conference and make presentations about MAPS. The conference was not as advertised; for more, see Jon Hanna’s article in the Winter 2005 Entheogen Review. |
| |
| Conference: Sheshamans - $970 |
| MAPS staffer Julia Onnie-Hay attended this conference, gave a presentation about MAPS, and ran a silent auction that raised over $500 for MAPS’ Women’s Entheogen Fund. Conference organizer Diane Darling also donated $1137 to MAPS from the profits of the conference. |
| |
| Erowid.org Website - $62,004 |
| MAPS is the fiscal sponsor for Erowid.org, an educational website focused on providing information about psychoactive plants and drugs. Erowid is the most frequently visited psychoactive drug information site on the web. Erowid does not accept advertising on its site, which could generate significant income, but prefers to provide information in a non-commercial, non-judgmental context. Erowid relies on donations to support staff costs. |
| |
| DEA/ALJ Lawsuit - $46,625 |
| $32,290 of this sum was for legal fees paid to DC law firm Jenner & Block, the lead law firm in MAPS and Prof. Craker’s lawsuit against the DEA for rejecting Prof. Craker’s application to DEA for a Schedule I license to create a MAPS-sponsored marijuana production facility at UMass-Amherst. The remaining sums were for travel and expenses for witnesses and for transcripts of the court proceedings. The first $100,000 of Jenner & Block’s fees were covered by Phil Harvey’s Liberty Project. The ACLU provided the assistance of Senior Attorney Allen Hopper pro bono. Additional pro bono legal services were provided by Emanuel Jacobowitz of the law firm Steptoe & Johnson. The legal costs of MAPS’ DEA ALJ lawsuit were also offset by a $35,500 grant from the Marijuana Policy Project. We’re awaiting the Judge’s ruling. (See article on page 13 and www.maps.org/mmj/DEAlawsuit.html) |
| |
| DEA/UMASS Cong. Sign on Letter - $13,298 |
| MAPS staffer Jag Davies and MAPS associates Kelly Burns, Abby Bair, J. F. and Michael McFadden worked on and off over a period of two months out of the Drug Policy Alliance office in Washington, D.C., lobbying members of the US House of Representatives to sign on to US Rep. John Olver’s letter to DEA in support of Prof. Craker. This effort, aided by local chapters of SSDP, NORML, ASA, and other localized drug policy reform organizations, yielded a total of 38 signatures from Congressional representatives. (See www.maps.org/mmj/DEAlawsuit.html#1) |
| |
| Event: MPP - $1,000 |
| Richard Wolfe donated $1,000 to MAPS for staffers Rick Doblin and Valerie Mojeiko to attend the Marijuana Policy Project’s gala at the Playboy Mansion, to meet with potential donors. |
| |
| Film: Grof - $5,393 |
| MAPS was fiscal sponsor for a film about Dr. Stan Grof and his work. MAPS allocated 100% of the funds to the project. |
| |
| Film: Shulgin/Littlefield - $50 |
| MAPS has served as fiscal sponsor for Canadian filmmaker Connie Littlefield’s documentary about Ann and Sasha Shulgin, which is still under production. |
| |
| MAPS Forum - $4,620 |
| This sum is the amount paid to Jon Frederick for maintaining and moderating the MAPS Forum. |
| |
| MAPS Staff Retreat - $400 |
| In February 2005, MAPS staffers spent a weekend in a rented house on Jewfish Key, a small island in Sarasota Bay. We discussed the re-vamping of the membership and sales office, job tasks, and the potential of re-location of the MAPS office to the San Francisco Bay Area. Matt Atwood, former director of SSDP and IDEAL Reform, joined us for the weekend, acting as a consultant by providing feedback about non-profit management and MAPS’ membership and sales procedures. This staff retreat contributed to the decision to re-locate the MAPS office to California. |
| |
| Safer Alternative For Enjoyable Recreation
(SAFER) - $75,175 |
| MAPS is fiscal sponsor for SAFER’s educational activities. SAFER sponsors harm reduction education at college campuses across the country, primarily in Colorado. |
| |
| Educational Video - $1415 |
| MAPS staffer Jag Davies wrote and directed the 20- minute educational video “Working with Difficult Psychedelic Experiences.” This project is intended for young adults and is part of MAPS’ harm reduction education agenda. It is now posted for viewing at www.maps.org/wwpe_vid |
| |
| Wo/Men’s Alliance for Medical Marijuana
(WAMM) - $4285 |
| MAPS is fiscal sponsor for WAMM, a Santa Cruz-based non-profit cooperative medical marijuana patient association. |
| |
| Women’s Entheogen Fund (WEF) - $18,200 |
| This fund was established to support and facilitate women’s involvement in psychedelic research. This year’s recipients were Sandra Karpetas ($5,000), Amelia Barlow ($5,000), Sylvia Thyssen ($5,000), and Fire Erowid ($2,500). An additional $700 was used to subsidize speakers for the SheShamans conference. |
| |
| Bulletin - $27,859 |
| Printing and mailing costs for the MAPS Bulletin, MAPS’ primary means of communication with its members. MAPS also sends the Bulletin for free as an educational tool to about 400 scientists, government officials, drug war prisoners, and influential academics. Even though the Bulletin has been available on the MAPS website for nearly a decade, the hard-copy issues of the Bulletin are still important to MAPS’ educational and communitybuilding mission. We’re also gradually expanding the use of our monthly e-mail updates as an inexpensive and quicker way to communicate with MAPS supporters. |
| |
| Internet - $6,460 |
| Internet access, both for servers hosting the MAPS website itself and for access for our office computers. |
| |
| Web Administration - $27,105 |
| Expenses paid to independent contractors for managing the security of the MAPS website and customizing software. The maps.org website is MAPS’ primary educational resource. It averaged over 3000 unique visitors per day during FY 05-06. |
| |
| Web Content - $3,847 |
| MAPS staff expenses related to formatting and posting documents for the website, updating content, and updating our page about psychedelic research projects around the world. |
| |
| Copies - $776 |
| Expenses for photocopies, which were minimal because we post documents on our website when possible. |
| |
| Information - $650 |
| This category is for books, subscriptions, and other educational materials needed for the MAPS staff. |
| |
| Fundraising - $5,311 |
| These expenses are for the MAPS online auction, several fundraising events, keyword advertisements with Yahoo and Google, and MAPS information tables at events. |
| |
| Phones - $11,660 |
| One consequence of staff, researchers, and volunteers spread out across the world is higher phone costs than we would like. Although we try to communicate via email whenever possible, there are many situations when phone conversations are necessary. |
| |
| Postal - $13,823 |
| Postal costs are for MAPS membership renewal mailings, shipping of MAPS merchandise, and MAPS mail communications all over the world. |
| |
| Conference Fees - $570 |
| Fees for MAPS staff to attend a few conferences. |
| |
| Professional Services - $6,285 |
| About half of this sum is for accounting services such as annual reports and payroll. The other half is for computer troubleshooting and consulting. |
| |
| Staff travel - $25,773 |
| As our international clinical research agenda has gained traction, with key Phase 2 MDMA/PTSD studies in Switzerland and Israel being initiated this year, and other research projects throughout the US, staff travel continued to increase. |
| |
| Salary, benefits & taxes - $211,737 |
| As with most non-profit organizations, MAPS’ salaries are lower than those for jobs in the private-sector with comparable skills and responsibilities. The lower salary is compensated for by the satisfaction of working on issues that have personal and social relevance. Over time, as MAPS grows as an organization, our goal is to pay competitive salaries. We also welcome and are benefiting from an increasing amount of donated labor of a highly skilled nature from people for whom salaries are not a necessity. This donated time is a crucial factor in MAPS’ ability to undertake the range of projects that it does and to implement its projects in a professional manner. The following salary information is for current salaries as of the end of 2006, and reflects both current compensation packages and staffing levels. As a result, the total is greater than the $211,737 spent in FY-2005-2006. I'm reporting the current salaries in order to give the most accurate picture at the time this financial report is received. MAPS President, Rick Doblin, earns $55,000 per year, with no benefits. Director of Operations and Clinical Research Associate, Valerie Mojeiko, earns a combination of salary and benefits valued at $51,100 (base salary $33,800), Director of Communications, Jag Davies, earns salary/benefits of $40,600 (base salary $29,120), Membership and Sales Manager, Sarah Hufford, earns salary/ benefits of $40,000 (base salary $27,040), Technology Specialist and Events Coordinator, Josh Sonstroem, earns salary/benefits of $35,800 (base salary $27,040). For half-time work, Director of Financial Operations, Nicole Tavernier-Luebcke, earns salary/benefits of $30,300 (base salary $18,720). |
| |
| Fees: Bank, etc - $4,258 |
| Corporate fees for state registration, wire transfer fees, other bank and credit card fees, etc. |
| |
| Equipment Rental - $2,494 |
| Pitney Bowes postal equipment for the office. |
| |
| Office Rent: Sarasota - $8259 |
| In the old Sarasota office, rent was quite inexpensive: about $600 per month. However, the staff had outgrown the office space and we needed to move to larger space that, in Sarasota, would have cost about $1200 a month. |
| |
| Office Rent: Love Creek - $12,000 |
| MAPS has incurred a raise in rent costs after moving to California in late May, now $3000 a month. Of that amount, $1200 is considered rent and $1800 is part of the benefits package for MAPS staff who live at the Love Creek facility. The $12,000 represents the deposit on the new Love Creek office. |
| |
| Office Supplies - $6,996 |
| Includes customized envelopes, various printed handouts, brochures, book flyers, and regular office supplies. |
| |
| Computer/Office Equipment - $4,704 |
| Three of the computers in the main MAPS office (iMacs originally purchased in FY 99-00) died during FY 05-06, and that, along with an increase in staff, spurred us to purchase four new late-model computers: two iBook laptops, a G4 iMac, and a Mac mini, the new server computer for our office network. Also furniture, copiers, scanners, etc. |
| |
| Books & Tapes Purchased for Resale - $7,462 |
| Merchandise that we re-sell. |
| |
| SM Hofmann/Chamberlain Portrait - $21,606 |
| Royalties and production expenses for 50 signed prints of a standard-size portrait of Albert Hofmann by Dean Chamberlain, and expenses for mailing. |
| |
| LG Hofmann/Chamberlain Portrait - $15,500 |
| Royalties and production expenses for 25 signed prints of a large portrait of Albert Hofmann by Dean Chamberlain, and expenses for mailing. |
| |
| Huxley/Chamberlain Portrait - $3,060 |
| Royalties and production expenses for 50 signed prints of a portrait of Laura Huxley by Dean Chamberlain, and expenses for mailing. |
| |
| Shulgin/Chamberlain - $4,749 |
| Royalties and production expenses for 50 signed prints of a portrait of Ann and Sasha Shulgin by Dean Chamberlain, and expenses for mailing. |
| |
| Alex Grey/Hofmann Portrait - $31,754 |
| Royalties and production expenses for 50 signed prints of Alex Grey‘s portrait of Albert Hofmann, and expenses for mailing. |
| |
| Any questions or comments about the financial aspects of MAPS are most welcome. We strive to use the funds donated to us in an efficient and strategic manner. With the continued support of MAPS members, and with the growth of MAPS’ membership base and research projects, MAPS has the unique potential to develop into the nonprofit psychedelic and medical marijuana pharmaceutical company that we are all envisioning together. |
| |
- Rick Doblin, Ph.D., MAPS President
|
|
|
|
|
|
